Next-Generation Sequencing (NGS) has revolutionized genomic research by enabling rapid, high-throughput analysis of DNA and RNA. This technology has transformed fields such as genetics, molecular biology, and personalized medicine, making it possible to sequence entire genomes, study gene expression, and detect genetic variations with unprecedented speed and accuracy. In this blog, we will explore how NGS works, its applications, key technologies, and how it is shaping the future of life sciences.
What is Next-Generation Sequencing (NGS)?
Next-Generation Sequencing, often referred to as high-throughput sequencing, refers to a collection of advanced techniques that allow the simultaneous sequencing of millions to billions of DNA or RNA molecules. Unlike traditional Sanger sequencing, which sequences a single DNA fragment at a time, NGS generates massive amounts of data in parallel, reducing time and cost while significantly increasing the volume of sequence information.
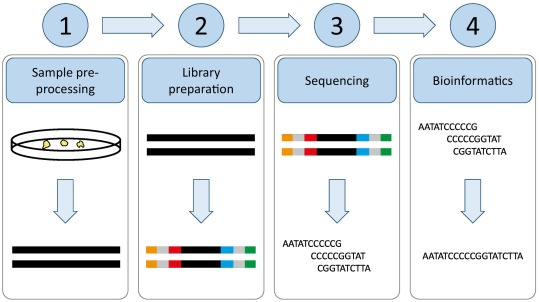
The key principle of NGS is the ability to fragment the DNA or RNA into smaller pieces, sequence these fragments, and then use bioinformatics tools to reassemble the data into a complete sequence or profile. This process enables the rapid analysis of entire genomes or specific regions of interest.
Key Steps in NGS
NGS typically involves several key steps:
- Library Preparation
In NGS, the DNA or RNA sample is first fragmented into smaller pieces. These fragments are then tagged with specific adaptors at their ends, forming a library of DNA molecules. The adaptors are necessary for binding the fragments to the sequencing platform and for amplification during the sequencing process. - Amplification
Once the library is prepared, the DNA fragments undergo amplification to create many copies of each fragment. This is typically done using a method called clonal amplification, such as bridge PCR or emulsion PCR. Amplification ensures that there is enough DNA for the sequencer to read and generate high-quality data. - Sequencing by Synthesis
In this step, the amplified DNA fragments are sequenced. Most NGS platforms use sequencing by synthesis (SBS), a process in which the DNA polymerase enzyme incorporates nucleotides into the growing DNA strand, and the incorporation of each nucleotide is detected and recorded. Platforms such as Illumina use fluorescently labeled nucleotides, where each base is read as it is incorporated into the growing DNA strand. - Data Analysis and Bioinformatics
After sequencing, the raw data generated by the sequencer is analyzed using bioinformatics tools. This involves assembling the short reads into a full sequence, aligning them to a reference genome, and identifying variations such as single nucleotide polymorphisms (SNPs), insertions, deletions, and other mutations. The complexity of this data requires advanced algorithms to make sense of the large volume of information.
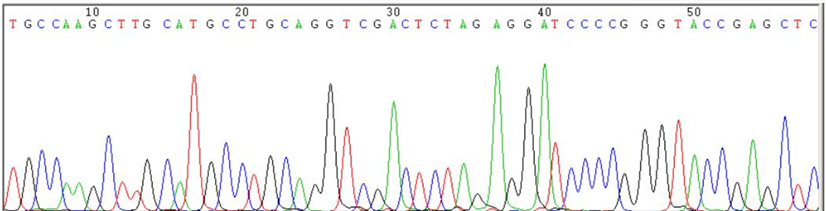
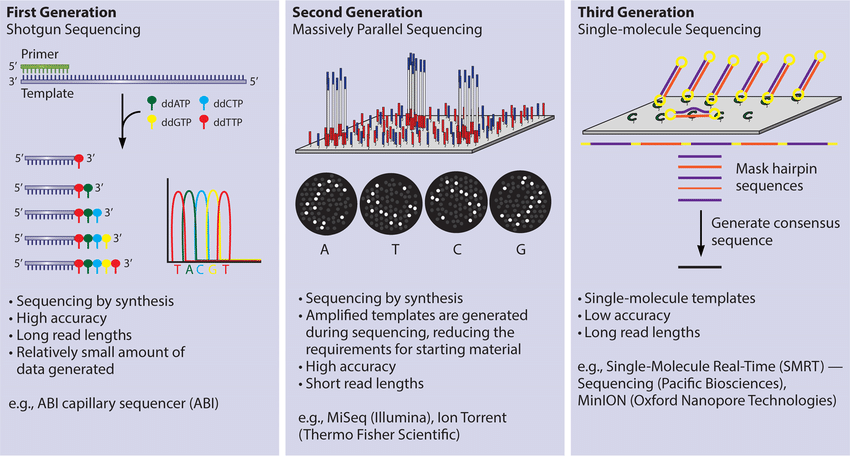
- First-generation sequencing's single read length is 800 bp;
- High accuracy, current gold standard for the sequencing;
- Molecular cloning is often performed after first-generation sequencing;
- Only one sequence can be measured at one time.
First Generation Sequencing price is cheap: 10-16 yuan/reaction. Generally 1 to 2 reactions are measured in conventional experiments.
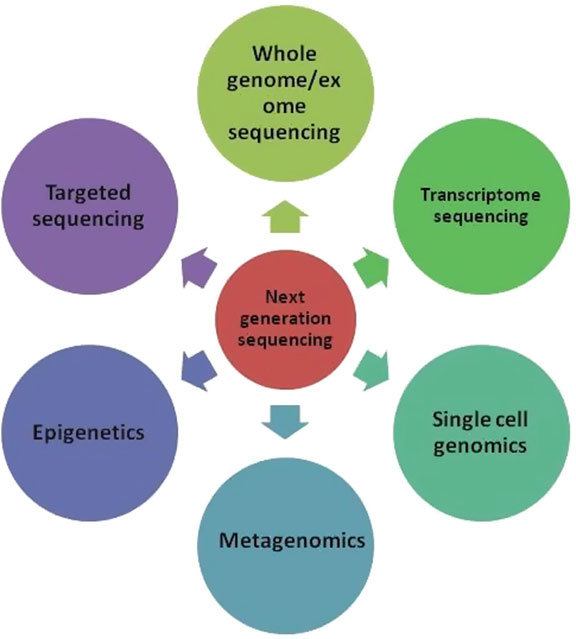
Applications of NGS
NGS has a wide range of applications across multiple fields of research and medicine. Below are some of the most important areas where NGS is making a significant impact:
- Whole Genome Sequencing (WGS)
WGS involves sequencing the entire genome of an organism, providing a comprehensive view of its genetic makeup. This approach is used to study genetic diversity, identify disease-causing mutations, and understand the evolution of organisms. NGS has reduced the time and cost of WGS, making it accessible for large-scale studies of human, animal, and microbial genomes. - Whole Exome Sequencing (WES)
WES focuses on sequencing the protein-coding regions of the genome, known as exons, which make up about 1% of the entire genome. This technique is valuable because most disease-causing mutations are found in exons. WES is widely used in clinical genetics to diagnose rare genetic disorders and uncover the genetic basis of diseases. - RNA Sequencing (RNA-Seq)
RNA-Seq is used to analyze the transcriptome—the complete set of RNA molecules in a cell. This includes both messenger RNA (mRNA), which codes for proteins, and non-coding RNAs, which regulate gene expression. RNA-Seq is critical for studying gene expression patterns, understanding disease mechanisms, and identifying potential therapeutic targets. - Targeted Sequencing
Targeted sequencing focuses on specific regions of the genome that are of interest, such as cancer-related genes or genes involved in inherited diseases. This approach allows for deep sequencing of these regions to detect rare mutations that might be missed by whole genome sequencing. NGS is widely used in cancer research to identify mutations driving tumorigenesis. - Metagenomics
NGS has transformed the study of microbial communities in various environments, including the human microbiome, by enabling researchers to sequence all the DNA present in a sample without the need for culturing. This approach, known as metagenomics, is used to study microbial diversity, understand ecological relationships, and discover new species of bacteria and viruses. - Epigenetics
NGS can be used to study epigenetic modifications, such as DNA methylation and histone modifications, which regulate gene expression without altering the underlying DNA sequence. Techniques like bisulfite sequencing allow researchers to map methylation patterns across the genome and understand their role in development, aging, and disease.
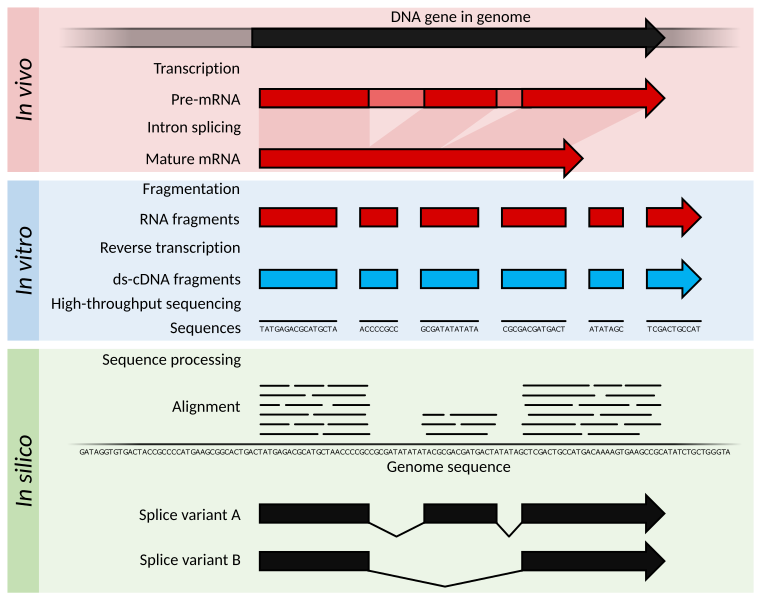
Key Technologies in NGS
Several NGS platforms are available, each with unique features that make them suitable for different applications. The most common NGS technologies include:
- Illumina Sequencing
Illumina is the most widely used NGS platform and is based on sequencing by synthesis (SBS). Illumina sequencers generate highly accurate data with read lengths of up to 300 base pairs (bp). The platform is versatile and can be used for a wide range of applications, from small targeted panels to whole genome sequencing. Illumina’s NovaSeq and MiSeq systems are popular choices for high-throughput and clinical applications. - Ion Torrent Sequencing
Ion Torrent technology detects changes in pH that occur as nucleotides are incorporated into the growing DNA strand. This approach is faster and less expensive than some other platforms but has shorter read lengths. Ion Torrent is ideal for targeted sequencing and amplicon sequencing, commonly used in cancer research and infectious disease studies. - Pacific Biosciences (PacBio) Sequencing
PacBio’s Single Molecule Real-Time (SMRT) sequencing is a long-read technology that allows for sequencing DNA fragments up to tens of thousands of base pairs in length. PacBio is especially useful for de novo genome assembly, structural variation detection, and sequencing of repetitive regions that are difficult to resolve with short-read technologies. - Oxford Nanopore Sequencing
Oxford Nanopore sequencing is another long-read technology that sequences DNA or RNA by detecting changes in electrical current as the nucleic acids pass through a nanopore. This platform is portable and can generate very long reads, making it suitable for fieldwork and rapid pathogen detection. Nanopore sequencing has been used in viral outbreak surveillance and environmental monitoring.

- The longest single read length of the second-generation sequencing is 300 bp, and the main sequencing in China is PE150.
- High accuracy.
- NGS library construction is often used for second-generation sequencing.
- Only multiple sequences can be measured at a time.
The sequencing price is medium, the price is 20-80 RMB/G, the experiment type is different, the sequencing amount is different, most 3 to 12G can meet the demand, a few technologies need to test 30-200 G.
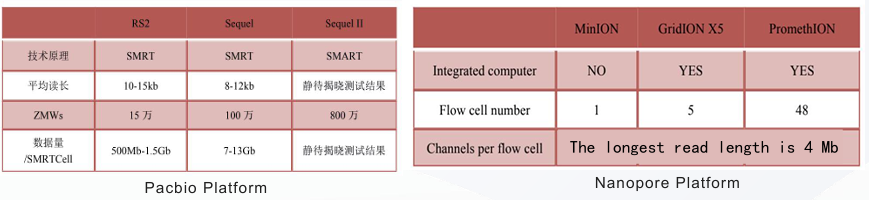
The single read length of third-generation sequencing is long;
Low accuracy, usually need the second-generation sequencing assistance;
Only multiple sequences can be measured at a time.
Three-generation Sequencing is more expensive, ranging from thousands to tens of thousands RMB.
Challenges and Limitations of NGS
While NGS has transformed genomics, it is not without its challenges. Some limitations include:
- Data Volume and Storage
NGS generates vast amounts of data, especially in applications like whole genome sequencing. Storing, managing, and analyzing these large datasets requires significant computational resources, bioinformatics expertise, and infrastructure. - Error Rates
Although NGS is highly accurate, errors can occur, particularly in low-quality samples or regions of the genome that are difficult to sequence, such as GC-rich regions. Long-read technologies like PacBio and Oxford Nanopore can have higher error rates compared to short-read platforms like Illumina. - Cost
While NGS has become more affordable, it still represents a significant cost for large-scale studies, especially when deep sequencing coverage is required. The cost of reagents, sequencing runs, and data analysis can add up, particularly for clinical applications. - Interpretation of Variants
Identifying genetic variants is relatively straightforward with NGS, but interpreting the functional significance of these variants is more challenging. Variants of uncertain significance (VUS) can complicate clinical decision-making, requiring additional research to determine their role in disease.

The Future of NGS
As NGS technology continues to evolve, we can expect even greater advances in genomics and personalized medicine. Some exciting developments include:
- Single-Cell Sequencing
Single-cell RNA sequencing (scRNA-seq) allows researchers to study gene expression at the individual cell level, providing insights into cellular heterogeneity, tissue development, and disease mechanisms. This technology is being used to explore cancer biology, immune responses, and tissue regeneration. - CRISPR and NGS
The combination of CRISPR gene-editing technology with NGS is opening new avenues for studying gene function, identifying off-target effects, and developing gene therapies. NGS can be used to validate CRISPR-mediated genome edits and track the outcomes of gene therapies in clinical trials. - Clinical Applications
NGS is already being used in clinical settings for cancer diagnostics, prenatal testing, and rare disease diagnosis. As the technology becomes more affordable and accessible, it is expected to play an even larger role in personalized medicine, helping to tailor treatments to individual patients based on their genetic profiles.
Next-Generation Sequencing has ushered in a new era of genomics, providing researchers and clinicians with powerful tools to explore the complexities of the genome and transcriptome. From whole genome sequencing to targeted panels and RNA sequencing, NGS offers unparalleled insights into biology and disease. As NGS technologies continue to evolve, they will undoubtedly drive further discoveries in life sciences and revolutionize healthcare.
While challenges such as data management, cost, and variant interpretation remain, ongoing advancements in sequencing platforms, bioinformatics, and single-cell